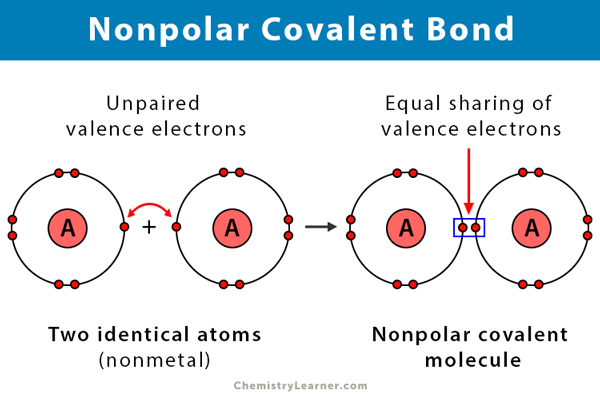
A covalent bond is formed when two atoms share electrons between them. In a nonpolar covalent bond, electrons are equally shared. This phenomenon happens when there is no difference in the electronegativities of the two atoms. That is, to say, identical pairs of atoms form a nonpolar covalent bond [1-4] .
Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. It is measured using a scale known as the Pauling scale and ranges from 0.7 to 4. The following table explains the different types of chemical bonds due to the electronegativity differences.
Nonpolar covalent
Slightly polar covalent

Nonpolar covalent bonds are essential in living things. For example, oxygen helps in the growth of the cells, and peptide bond joins together chains of amino acids, which are involved in the construction of the DNA.
Here are some facts about the nonpolar covalent bond.
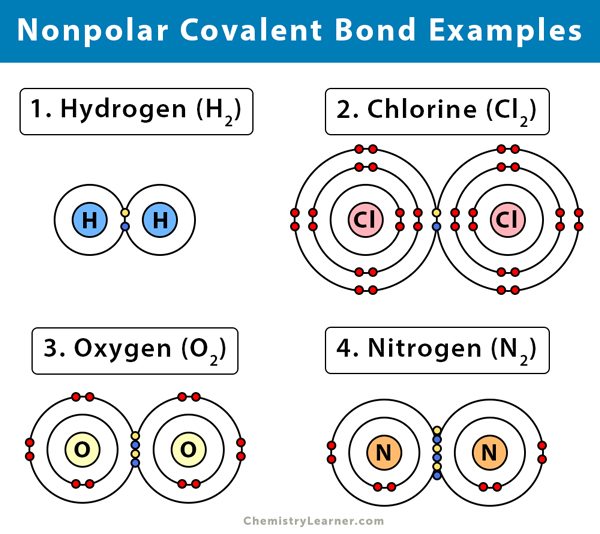
The substances that have nonpolar bonds and their molecular formulae are given below [2-4] .
Hydrogen (H2)
Take the example of a hydrogen molecule. Because the nuclei of each hydrogen (H) atom contains protons, the electrons in the bond are attracted to the nuclei. However, because the two atoms involved in a single covalent bond are both H atoms, each nucleus attracts the electrons by the same amount. Thus, the electron pair is equally shared by the two atoms.
Some nonpolar molecules are containing polar covalent bonds. In these molecules, the orientation of the various polar bonds is such that their polarities cancel each other. Below is a list of such molecules with their formulae.